Table Salt Is A Compound Or Element
bustaman
Dec 05, 2025 · 12 min read
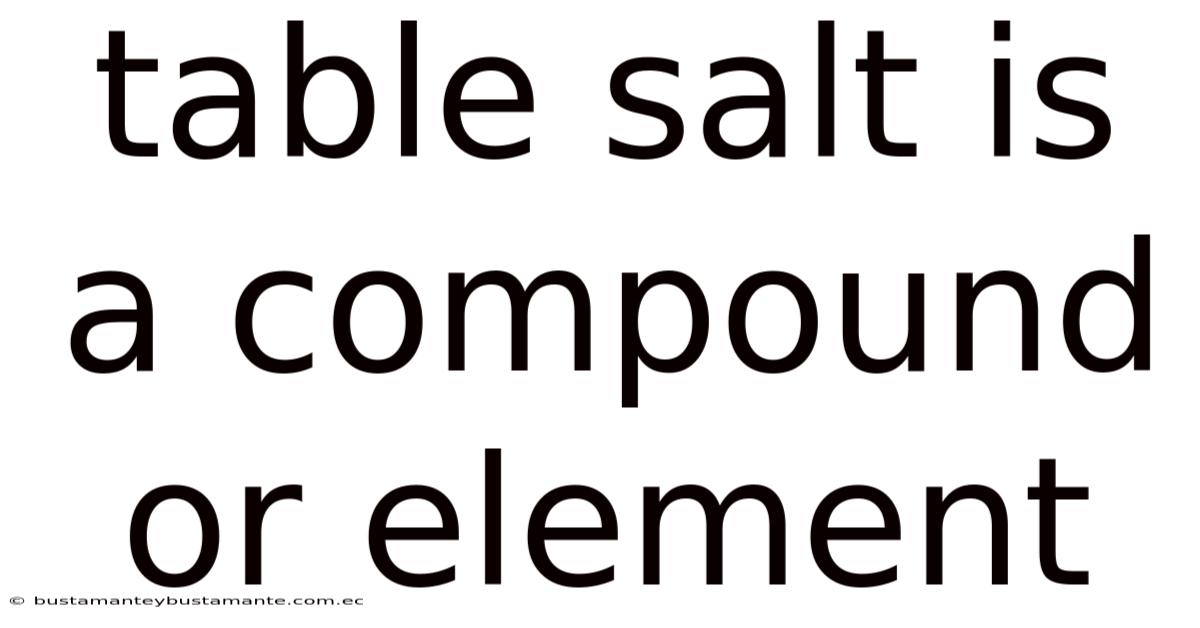
Table of Contents
Imagine you're in the kitchen, ready to whip up your favorite dish. You reach for the salt shaker, that unassuming container holding tiny white crystals, and sprinkle a dash into your simmering sauce. But have you ever paused to consider what exactly that salt is? Is it a fundamental element, like the gold in jewelry, or something more complex? The answer lies in the fascinating world of chemistry.
The question of whether table salt is a compound or an element is a common one, particularly for those new to the sciences. In short, table salt is a compound. To understand why, we need to delve into the basics of chemistry and explore the composition of this ubiquitous substance. This article will break down the nature of table salt, its chemical makeup, and why it’s crucial to our daily lives.
Main Subheading
To understand why table salt is classified as a compound, it’s essential to first define what elements and compounds are. An element is a pure substance that consists of only one type of atom. These atoms cannot be broken down into simpler substances by chemical means. Examples of elements include hydrogen (H), oxygen (O), sodium (Na), and chlorine (Cl). Each element is defined by its unique atomic number, which represents the number of protons in the nucleus of its atoms.
On the other hand, a compound is a substance formed when two or more elements are chemically bonded together in a fixed ratio. This bonding occurs through the sharing or transfer of electrons between atoms, resulting in a new substance with properties that are distinct from those of its constituent elements. For instance, water (H2O) is a compound formed from hydrogen and oxygen, and its properties are vastly different from either hydrogen or oxygen gas. The formation of a compound involves a chemical reaction, which rearranges atoms to form new molecules or ionic lattices.
Comprehensive Overview
Defining Elements and Compounds
The distinction between elements and compounds is fundamental to chemistry. Elements are the simplest forms of matter and serve as the building blocks for all substances. They are organized in the periodic table, a chart that arranges elements based on their atomic number and chemical properties. Each element has a unique symbol, such as Na for sodium and Cl for chlorine, making it easy to represent them in chemical formulas and equations.
Compounds, however, are more complex. They are created when elements combine through chemical bonds. These bonds can be covalent, where electrons are shared between atoms, or ionic, where electrons are transferred from one atom to another. The type of bond that forms depends on the electronegativity of the atoms involved. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond.
The Chemical Composition of Table Salt
Table salt, scientifically known as sodium chloride (NaCl), is a prime example of a compound. It is formed through the ionic bonding of sodium (Na) and chlorine (Cl) atoms. Sodium is a highly reactive metal that readily loses an electron to achieve a stable electron configuration, forming a positively charged sodium ion (Na+). Chlorine, conversely, is a highly reactive nonmetal that readily gains an electron to achieve a stable electron configuration, forming a negatively charged chloride ion (Cl-).
When sodium and chlorine atoms come into contact, sodium donates its outermost electron to chlorine. This electron transfer results in the formation of Na+ and Cl- ions, which are oppositely charged and therefore attract each other strongly. This electrostatic attraction is what forms the ionic bond between sodium and chlorine, creating the compound sodium chloride. The resulting compound has a crystal lattice structure, where Na+ and Cl- ions are arranged in an alternating pattern, forming a three-dimensional network.
Properties of Sodium Chloride
The properties of sodium chloride are markedly different from those of its constituent elements, sodium and chlorine. Sodium is a soft, silvery-white metal that reacts violently with water, while chlorine is a greenish-yellow gas that is highly toxic. When these elements combine to form sodium chloride, they produce a stable, white crystalline solid that is essential for life.
Sodium chloride has a high melting point (801°C) and a high boiling point (1413°C), reflecting the strength of the ionic bonds holding the crystal lattice together. It is also highly soluble in water, dissociating into Na+ and Cl- ions in solution. This property is crucial for its biological functions, as these ions play essential roles in maintaining fluid balance, nerve function, and muscle contraction in living organisms.
Historical and Geological Context
The formation of sodium chloride has significant historical and geological context. Naturally, sodium chloride is found in vast underground deposits and in seawater. These deposits were formed over millions of years through the evaporation of ancient seas. As seawater evaporates, the concentration of dissolved salts increases until they begin to precipitate out of solution, forming layers of salt crystals.
Historically, salt has been a valuable commodity, used for preserving food, flavoring dishes, and as a form of currency. Ancient civilizations, such as the Romans and Egyptians, recognized the importance of salt and developed sophisticated methods for extracting and trading it. The term "salary" is derived from the Latin word "salarium," which refers to the salt ration given to Roman soldiers. This historical context underscores the significance of sodium chloride in human civilization.
The Role of Salt in the Human Body
Beyond its culinary uses, sodium chloride plays a crucial role in maintaining human health. The human body requires a certain amount of sodium and chloride ions to function properly. These ions are involved in regulating fluid balance, maintaining blood pressure, and transmitting nerve impulses. Sodium ions, in particular, are essential for the proper functioning of nerve and muscle cells.
Sodium and chloride ions help maintain the osmotic balance of body fluids, ensuring that cells have the right concentration of water and electrolytes. They also play a role in the transport of nutrients and waste products across cell membranes. Additionally, chloride ions are a key component of gastric acid (hydrochloric acid, HCl), which is essential for digestion in the stomach.
Trends and Latest Developments
The Debate on Salt Consumption
Despite its essential role in human health, excessive salt consumption has been linked to various health problems, including high blood pressure, heart disease, and stroke. As a result, there has been a growing emphasis on reducing salt intake in the modern diet. Health organizations, such as the World Health Organization (WHO) and the American Heart Association (AHA), recommend limiting sodium intake to less than 2,300 milligrams per day for adults.
The debate on salt consumption involves understanding the balance between the essential need for sodium and the potential risks of excessive intake. Many processed foods are high in sodium, contributing significantly to overall sodium intake. Public health campaigns aim to educate consumers about the sodium content of foods and encourage them to make healthier choices.
Alternative Salts and Their Composition
In recent years, there has been an increasing interest in alternative salts, such as sea salt, Himalayan pink salt, and kosher salt. These salts are often marketed as being healthier or having a superior flavor compared to regular table salt. However, chemically, all these salts are primarily sodium chloride. The main differences lie in their trace mineral content and processing methods.
Sea salt is produced by evaporating seawater and contains trace amounts of minerals such as magnesium, calcium, and potassium. Himalayan pink salt is mined from salt deposits in the Himalayas and gets its pink color from iron oxide. Kosher salt is a coarse-grained salt that is favored by chefs for its ability to draw moisture out of food. While these alternative salts may offer subtle differences in flavor and mineral content, they are still primarily sodium chloride and should be consumed in moderation.
Technological Advances in Salt Production
Technological advances continue to improve the efficiency and sustainability of salt production. Modern salt production methods include vacuum evaporation, solution mining, and solar evaporation. Vacuum evaporation involves boiling brine under reduced pressure to accelerate evaporation. Solution mining involves injecting water into underground salt deposits to dissolve the salt and then pumping the brine to the surface for evaporation. Solar evaporation relies on the natural evaporation of seawater or brine in large ponds.
These technological advances aim to reduce energy consumption, minimize environmental impact, and improve the quality of the salt produced. Additionally, research is ongoing to develop more sustainable methods for salt production, such as capturing and reusing waste heat and reducing water consumption.
Tips and Expert Advice
Reading Food Labels for Sodium Content
One of the most effective ways to manage your salt intake is to read food labels carefully. The Nutrition Facts label on packaged foods provides information about the sodium content per serving. Pay attention to the serving size and the amount of sodium listed. Look for foods that are labeled as "low sodium," "reduced sodium," or "no salt added."
It's also important to be aware of hidden sources of sodium in foods. Many processed foods, such as canned soups, frozen dinners, and snack foods, are high in sodium. Even foods that don't taste particularly salty can contain significant amounts of sodium. By becoming a savvy label reader, you can make informed choices about the foods you eat and reduce your overall sodium intake.
Cooking at Home to Control Salt Intake
Cooking at home is another great way to control your salt intake. When you cook your own meals, you have complete control over the ingredients and seasonings you use. You can reduce the amount of salt you add to recipes and experiment with other flavor enhancers, such as herbs, spices, and citrus juices.
Start by gradually reducing the amount of salt you use in your favorite recipes. You may not even notice the difference, especially if you focus on adding other flavorful ingredients. Try using fresh herbs like basil, oregano, and thyme to add depth of flavor to your dishes. Spices like garlic powder, onion powder, and paprika can also enhance the taste of your food without adding sodium. A squeeze of lemon or lime juice can brighten up many dishes and add a refreshing zing.
Understanding Salt Substitutes
For those looking to further reduce their sodium intake, salt substitutes can be a useful tool. Salt substitutes typically contain potassium chloride instead of sodium chloride. Potassium chloride has a similar taste to sodium chloride but does not raise blood pressure in the same way. However, it's important to consult with a healthcare professional before using salt substitutes, especially if you have kidney problems or are taking certain medications.
While salt substitutes can help reduce sodium intake, they may not be suitable for everyone. Some people find that potassium chloride has a slightly bitter or metallic taste. It's also important to use salt substitutes in moderation, as excessive potassium intake can also have adverse health effects. Experiment with different salt substitutes to find one that you enjoy and that fits your dietary needs.
Balancing Electrolytes During Exercise
For athletes and individuals who engage in intense physical activity, maintaining proper electrolyte balance is crucial. During exercise, the body loses electrolytes, including sodium and chloride, through sweat. These electrolytes need to be replenished to prevent dehydration, muscle cramps, and other performance-limiting conditions.
Sports drinks are often formulated to contain electrolytes, including sodium and chloride, to help replenish what is lost during exercise. However, it's important to choose sports drinks wisely, as some can be high in sugar and calories. Alternatively, you can make your own electrolyte drink by adding a pinch of salt to water or consuming electrolyte-rich foods such as bananas, coconut water, and leafy green vegetables.
Salt in Food Preservation
Historically, salt has been used as a method of food preservation. Its ability to draw out moisture inhibits the growth of bacteria, fungi, and other microorganisms that cause spoilage. This is why salting is used in the preservation of meats, fish, and vegetables. While modern refrigeration and other preservation methods have reduced our reliance on salt for this purpose, it is still used in the curing process of many foods.
Foods like ham, bacon, and sauerkraut rely on salt for their unique flavors and preservation qualities. The high salt content not only preserves these foods but also contributes to their distinctive textures and tastes. However, it is important to consume these foods in moderation due to their high sodium content.
FAQ
Q: Is sea salt healthier than table salt? A: Sea salt and table salt are both primarily sodium chloride. Sea salt may contain trace minerals, but the amounts are usually insignificant. The main difference lies in taste and texture, not health benefits.
Q: Can I use salt substitutes to reduce my sodium intake? A: Salt substitutes, which typically contain potassium chloride, can help reduce sodium intake. However, they may not be suitable for everyone, especially those with kidney problems or those taking certain medications. Consult with a healthcare professional before using salt substitutes.
Q: How much salt should I consume daily? A: Health organizations recommend limiting sodium intake to less than 2,300 milligrams per day for adults. This is equivalent to about one teaspoon of table salt.
Q: What are the symptoms of excessive salt intake? A: Symptoms of excessive salt intake can include high blood pressure, bloating, and swelling in the ankles and feet. Over time, chronic high salt intake can increase the risk of heart disease and stroke.
Q: Are there any foods that naturally contain high amounts of sodium? A: Yes, some foods naturally contain high amounts of sodium, such as shellfish, seaweed, and celery. However, most of the sodium in our diets comes from processed foods and added salt.
Conclusion
So, is table salt a compound or an element? The answer is definitively a compound. It is the result of a chemical bond between the element sodium (Na) and the element chlorine (Cl), forming sodium chloride (NaCl). Understanding the nature of salt, its chemical composition, and its role in our lives is essential for making informed dietary choices and appreciating the chemistry that underlies our everyday experiences. By being mindful of our salt consumption and exploring alternative ways to enhance flavor, we can enjoy our meals while maintaining a healthy lifestyle.
Now that you're armed with this knowledge, take a closer look at the food labels in your pantry, experiment with new recipes that emphasize herbs and spices over salt, and share this article with friends and family. Let's spread awareness about the importance of understanding the chemistry of everyday substances and making informed choices for our health. What are your favorite ways to reduce salt intake without sacrificing flavor? Share your tips in the comments below!
Latest Posts
Latest Posts
-
What Characteristics Of Living Things Do Viruses Have
Dec 05, 2025
-
What Is Group 18 On The Periodic Table
Dec 05, 2025
-
Which Membrane Is Composed Of Epithelium
Dec 05, 2025
-
How Do You Find The Angle Of Rotation
Dec 05, 2025
-
German Monk Who Started The Protestant Reformation
Dec 05, 2025
Related Post
Thank you for visiting our website which covers about Table Salt Is A Compound Or Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.